Business
Most Common Elements in the Earth’s Crust
Elements are the most fundamental kind of stuff. Chemical techniques cannot split the contents of the elements into simpler basic units of matter since they are made up of uniform atoms. All other substances are compounds, alloys, or mixes. Water, for example, has a molecular structure that is made up of Hydrogen and Oxygen components.

What is The Earths Crust?
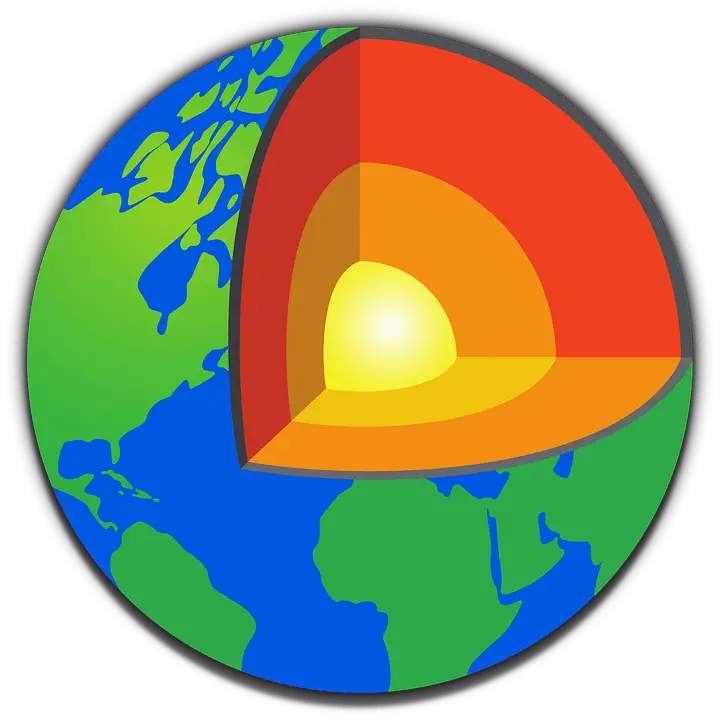
Earth Crust refers to the Earth’s outer crust (Stone Sphere). Elements, compounds, alloys, and mixes are also constituents of the Earth’s crust.
The Earth’s crust accounts for 1% of the planet’s total volume. The most prevalent elements in the Earth’s crust are oxygen, silicon, iron, and aluminum, accounting for 88.1 percent of its mass.
Nobody has ever traveled to the core of the Earth. We’ve got about a third of the way to the mantle.
Elements in the Earth’s crust furnish all of the basic building blocks for humanity.
Although the crust is the origin of everything we uncover, mine, refine, and build, it merely touches the surface of our planet.
The three common Elements of Earth’s Crust
1. Oxygen: In the Earth’s crust, it is the most plentiful element.
2. Hydrogen: It is just the tenth most prevalent element in the Earth’s crust.
3. Aluminum: The most abundant metal in the Earth’s crust is aluminum.
The Earth’s crust comprises around 95% igneous and metamorphic rocks, 4% shale, 0.75 percent sandstone, and 0.25 percent limestone.
88.1 percent of the Earth’s crust comprises the elements oxygen, silicon, aluminum, and iron, with the remaining 11.9 percent made up of 90 other elements.
Let’s look at the ten elements that make up the Earth’s crust.
1. Oxygen (Quantity: 46.1%)
In the Earth’s crust, it is the most common element. It accounts for around 46 percent of the Earth’s crust.
Because oxygen is a highly reactive element, and it is generally found as a compound. For example, iron rust is formed when iron and oxygen combine. Combustion reactions can also occur due to the interaction of chemicals with oxygen.
Oxygen is a highly reactive element that reacts with other ingredients to generate oxides. Minerals such as granite and quartz (silicon oxides), rust (iron oxides), and limestone are examples of common oxides (oxide of calcium and carbon).
Liquid oxygen is highly flammable and is used as a fuel, but the combination of oxygen and acetylene produces a flame hot enough for welding and metal melting. Furthermore, most biological life on Earth requires oxygen to survive. It is one of the essential components of all living beings.
2. Silicon (Quantity: 28.2%)
Silicon is the second most abundant element. Silicon is a critical semiconductor utilized in producing electronics and computer chips.
Most sand comprises silicon dioxide, which is why it is so abundant in the Earth’s crust. Sand is primarily composed of silicon-based minerals and rocks.
Silicon dioxide, made of silicon and oxygen, is one of the most frequent of these compounds.
3. Aluminum (Quantity: 8.23%)
It accounts for around 8% of the Earth’s crust. However, aluminum is seldom encountered in its elemental form due to its great affinity for oxygen. Aluminum oxide, aluminum hydroxide, and potassium aluminum sulfate are common aluminum compounds.
Though it is the most abundant metal, it is always found in complex form and never in its natural condition.
Aluminum in its pure form is nearly hard to locate in the Earth’s crust. Aluminum oxide is the most common Aluminum compound. Aluminum shapes and alloys are frequently utilized, particularly in cookware and aircraft.
4. Iron (Quantity: 5.63%)
Iron constitutes 5% of the Earth’s crust. This ingredient, which people have been digesting and using for thousands of years, is significant enough to launch and conclude an era in human progress.
The minerals hematite and magnetite are the primary sources of iron. Over 90% of all metals mined are iron, used primarily to manufacture steel, a carbon-iron alloy. Iron is also a necessary nutrient in the human body.
Steel is formed when iron and carbon combine to make one of the most often used metals, utilized in everything from small household goods to bridges and structures. Iron is also required for biological survival. It is a necessary component of human blood and plant chlorophyll.
5. Calcium (Quantity: 4.15%)
Calcium compounds are commonly utilized for supplementing in the food and pharmaceutical sectors. Calcium makes up around 4% of the Earth’s crust.
It is also a highly reactive element. It cannot be found in nature in its pure form. For example, it may react with water and oxygen without being triggered.
Calcium is a smooth, silvery-white alkaline earth metal in its pure elemental state. It is never discovered in nature in its solitary form but rather in compounds. Calcium compounds are found in several minerals, including limestone (calcium carbonate), gypsum (calcium sulfate), and fluorite (calcium fluoride).
6. Sodium (Quantity: 2.36%)
Sodium is the sixth most common element, and it is also a highly reactive element. It is nearly hard to find the pure, as it is with other reactive components. When poured into water, it reacts so fast that explosions occur.
For most people, sodium is connected with rock salt – sodium chloride. Because sodium is particularly water-soluble, it is one of the most common dissolved elements found in the ocean, and saltwater bodies frequently form sodium chloride, or salt deposits, especially when the body of water has dried up.
Sodium is also a crucial element for animals and humans since it helps biological life maintain sufficient fluid equilibrium, affecting neurons and muscle fibers.
7. Magnesium (Quanity:2.33%)
It is most widely utilized in the aviation sector, pharmaceuticals, and photography. However, its most significant function in the human body is bone and muscle formation, and it also plays an active part in the neurological system.
It is the seventh most common element. The metal does not exist as a free element but rather in conjunction with other details such as oxygen, calcium, and carbon. Dolomite is a magnesium-containing mineral.
8. Potassium (Quantity: 2.09%)
Potassium is found naturally in potash and minerals such as sensuality, sylvite, and polyhalite. However, potassium chloride, which is used in fertilizers and other products, and potassium carbonate, used in soaps and some types of glass, are the most frequent potassium compounds.
It is another essential nutrient for our bodies. Its primary purpose in the human body is to play a role in the neurological system. It is reactive to oxygen and hydrogen in its pure state, and it can ignite in water or open air.
9. Titanium (Quantity: 0.565%)
It is 45 percent lighter than steel and has the same strength and durability. In addition, because of its exceptional resistance to saltwater, its alloys are particularly useful in ships with metal hulls.
As a result, humans employ it in various applications, from airplanes to human prosthetic joints.
It is also very resistant to temperature swings and high temperatures. As a result, it is commonly required in the space sector.
10. Hydrogen (Quantity: 0.140%)
Hydrogen is the lightest element. Therefore, it is most usually encountered as a gas.
When burned, it is the cleanest fuel because only water is produced, and it is widely employed in fuel technologies today.
Hydrogen contains several abundant chemicals on Earth, both in nature and in artificial applications. Hydrogen is, of course, a fundamental component of water, H2O. Still, it is also present in common molecules such as ammonia, methane, hydrogen peroxide, and sugar, all of which are readily available to people.
Rest of the elements: 0.48%
Is it a difficult task to dig into the Earth’s Crust?
Despite Jules Verne’s fantasy, nobody has ever been to the core of the Earth.
The most bottomless hole ever drilled by humans is around 12 km (7.5 miles) below the Earth’s surface, nearly one-third of the way to the Earth’s mantle. It took 20 years to get to this remarkable depth.
Although humanity is constantly discovering discoveries and aiming for the heavens, there is still plenty to learn about the planet we live on.
Conclusion:
When you consider the difficulty of obtaining some of these elements towards the bottom of the list, it’s easy to see why silver and gold are so precious. But, unfortunately, they’re pretty tricky to come by in the larger scheme of things.
Read More Ten Interesting Facts About Earth





































